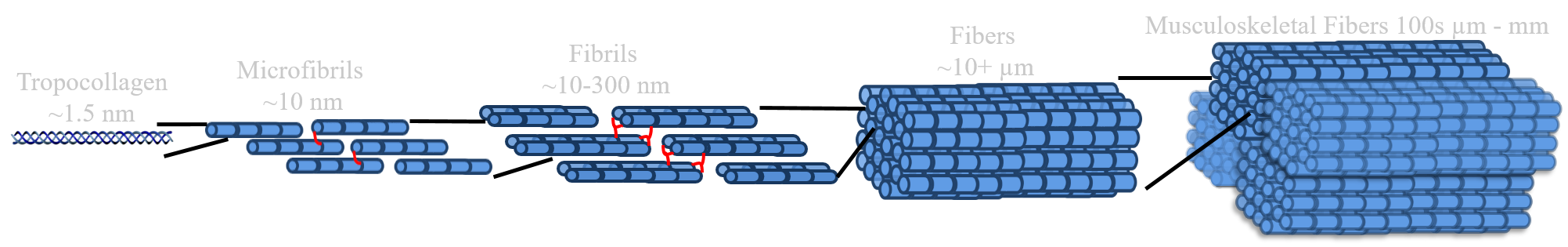
Musculoskeletal menisci, tendons, and ligaments aid in movement while providing joint support and stability. These tissues are composed of large hierarchically organized collagen fibers, ranging from 10 nm - 10 mm in diameter. These collagen fibers provide the strength necessary for function. Injuries disrupt this collagen organization resulting in lost of function, pain, decreased mobility, and over 1.6 million surgeries a year in the US. Currently these tissues are replaced by autograft or allograft transplants, which have limited availability, risk of immune rejection, and donor site morbidity. Engineered replacements are a promising alternative, but often lack organized collagen necessary for successful long-term function.
Our lab is working to better understand how musculoskeletal tissues produce these intricate collagen fibers in order to create functional replacements and drive repair in vivo.
Our lab is working to better understand how musculoskeletal tissues produce these intricate collagen fibers in order to create functional replacements and drive repair in vivo.
Focus Areas
Collagen Fiber Development
Bone Interface Engineering
Effects of Ageing and Injury
Collagen Fiber Development
We have developed a culture system capable of guiding cells to produce native size 20-40 µm collagen fibers by 6 weeks of culture for meniscus, tendon, and ligament tissues. Interestingly, each cell type (meniscal fibrochondrocytes, tenocytes, and ligament fibroblasts) create significantly different sized collagen fibers which match their respective native tissue, suggesting cells have an intrinsic ability to regulate fiber formation. Currently, we are investigating how cells sense their surroundings and regulate this collagen fiber formation in an effort to reproduce specific fiber geometries across multiple tissues both in vitro and in vivo. This work is funded by an NIH R01, in which we are currently investigating the role of FAK, TRPV4, and Piezo1 sensing in hierarchical fiber formation.
Using this culture system we have also begun to develop larger hierarchical organizations, with fibers grouping into 300-400 µm wide collagen bundles. We are using chemical and developmental-inspired mechanical stimulation to further drive fiber development to larger hierarchical sizes in an effort to produce engineered tissues with fibers matching the mm-cm wide native musculoskeletal fiber organizations. Our work investigating the effect of mechanical stimulation on further fiber development is funded by the Interdisciplinary Rehabilitation Engineering Research Career Development Program (IREK12) and a NSF CAREER award.
Using this culture system we have also begun to develop larger hierarchical organizations, with fibers grouping into 300-400 µm wide collagen bundles. We are using chemical and developmental-inspired mechanical stimulation to further drive fiber development to larger hierarchical sizes in an effort to produce engineered tissues with fibers matching the mm-cm wide native musculoskeletal fiber organizations. Our work investigating the effect of mechanical stimulation on further fiber development is funded by the Interdisciplinary Rehabilitation Engineering Research Career Development Program (IREK12) and a NSF CAREER award.
Bone Interface Engineering
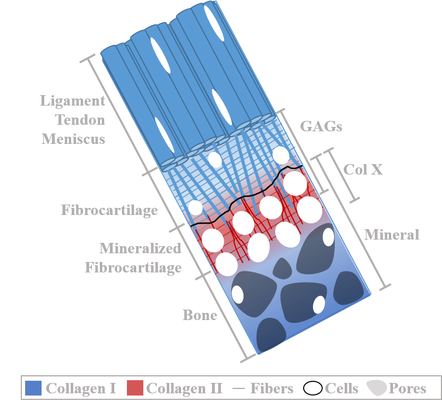 Complex structure and organization of the Enthesis which anchors tendons, ligaments and menisci to bone throughout the body
Complex structure and organization of the Enthesis which anchors tendons, ligaments and menisci to bone throughout the body
Tendons, ligaments, and menisci are anchored to bone by a structurally complex tissue called the enthesis. The enthesis translates loads from stretchy elastic soft tissues to stiff bone via a compliant fibrocartilaginous region. Entheses are able to translate these loads due to an intricate zonal organization with gradients in collagen fiber organization, mineralization, and cell phenotypes. Currently, these gradients are not recreated in soft tissue-to-bone healing or engineered replacements. In fact, 20-94% of rotator cuff and ACL repairs fail, primarily at the enthesis, due to a lack of regeneration. There is a need to drive enthesis regeneration in engineered replacements to create long-lasting functional tissue.
We believe recapitulating the developmental process is a promising means to produce enthesis gradients. Our novel culture system which guides cells to produce native sized collagen fibers for tendon, ligament, and meniscus, is also well suited for enthesis development, producing a unique interface between the aligned midsection and the compressive clamps. Using this culture system we have found we can create ligament-to-bone tissue similar to what is seen in animals and humans just after birth. Currently we are investigating whether applying developmentally inspired mechanical loads to this system can further drive the complex gradients in organization, composition, and cell phenotype. We are also investigating how these mechanical cues regulate zonal stem cell differentiation for enthesis development. Our goal is to create a functional enthesis replacement while gaining an understanding of what drives enthesis organization in an effort to be able to recreate it after injury for tissues throughout the body.
This work is currently funded by the Alliance for Regenerative Rehabilitation Research and Training (AR3T) center and the Orthoregeneration (ON) Foundation.
We believe recapitulating the developmental process is a promising means to produce enthesis gradients. Our novel culture system which guides cells to produce native sized collagen fibers for tendon, ligament, and meniscus, is also well suited for enthesis development, producing a unique interface between the aligned midsection and the compressive clamps. Using this culture system we have found we can create ligament-to-bone tissue similar to what is seen in animals and humans just after birth. Currently we are investigating whether applying developmentally inspired mechanical loads to this system can further drive the complex gradients in organization, composition, and cell phenotype. We are also investigating how these mechanical cues regulate zonal stem cell differentiation for enthesis development. Our goal is to create a functional enthesis replacement while gaining an understanding of what drives enthesis organization in an effort to be able to recreate it after injury for tissues throughout the body.
This work is currently funded by the Alliance for Regenerative Rehabilitation Research and Training (AR3T) center and the Orthoregeneration (ON) Foundation.
Effects of Aging & Injury
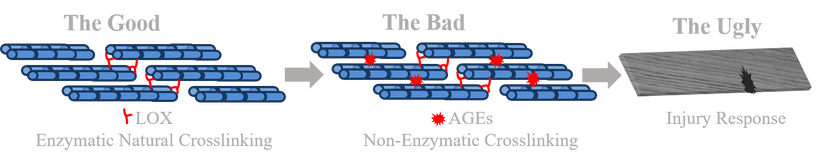
There is a clear link between increasing age and injuries in musculoskeletal tissues. In fact, it is reported 50% of individuals over the age of 18 in the US will suffer a musculoskeletal injury, with this number increasing to 75% by age 65. These injuries have minimal chance of healing, leading to reduce mobility, pain, and replacement. Many mechanisms have been suggested to contribute; however the most prominent mechanism in musculoskeletal tissues, due to their high reliance on collagen, is believed to be advanced glycation end-products (AGEs). Musculoskeletal menisci, tendons, and ligaments are dominated by hierarchically organized collagen fibers. With age, AGEs produce non-enzymatic crosslinks within the collagen, resulting in stiffer tissues more prone to injury. It is believed AGEs drive the progression of many connective tissue injuries and diseases which develop with age, including arthritis, osteoarthritis, tendinopathy, atherosclerosis, and osteoporosis. However, the reported effect of AGEs in musculoskeletal tissues have been inconsistent. Little is known how AGEs impact matrix mechanics, cell-matrix interactions, and matrix remodeling.
We recently developed a culture method to induce a wide range of AGE crosslinks that match human levels for various tissues, ages, and diseases, while maintaining cell viability. We are currently using this method to investigate the effect of AGEs on cells and cell-matrix interactions at each hierarchical level of collagen in order to obtain a better understanding of how AGEs effect tissues throughout the body with various degrees of collagen fiber organization. We are also investigating therapeutic means of reducing AGEs with mechanical loading. Our long term goal is to unravel the mechanism of aging in musculoskeletal tissues in an effort to develop new therapeutic targets and treatments to reduce and repair age-related injuries. This work is currently supported by a VCU CCTR Endowment Grant.
We recently developed a culture method to induce a wide range of AGE crosslinks that match human levels for various tissues, ages, and diseases, while maintaining cell viability. We are currently using this method to investigate the effect of AGEs on cells and cell-matrix interactions at each hierarchical level of collagen in order to obtain a better understanding of how AGEs effect tissues throughout the body with various degrees of collagen fiber organization. We are also investigating therapeutic means of reducing AGEs with mechanical loading. Our long term goal is to unravel the mechanism of aging in musculoskeletal tissues in an effort to develop new therapeutic targets and treatments to reduce and repair age-related injuries. This work is currently supported by a VCU CCTR Endowment Grant.
Proudly powered by Weebly